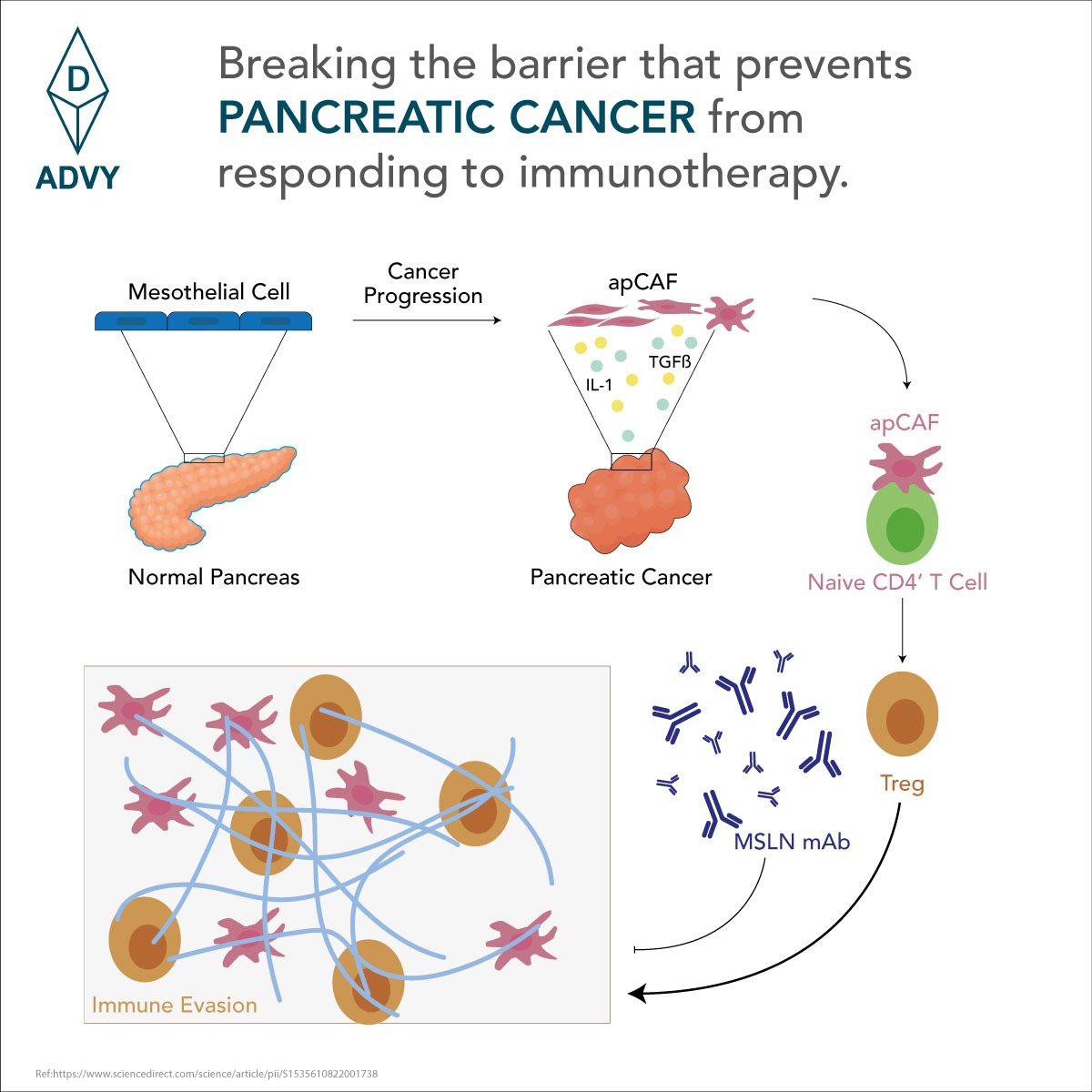
Background and aim:
Source: FT article
Disclaimer: Views expressed are solely of the author and have no bearing on Advy Chemical Pvt Ltd.
Every individual and enterprise is working towards leaving behind a ‘brighter, greener’ future and harnessing research to identify ways to overcome the challenges we are facing.

The In-Vitro Diagnostics (IVD) industry, a lesser known sub-segment of the broader healthcare industry has set out on a similar path.The thoughtful and well-identified gap in medicine published in the re-printed FT article, while originally featured in 2015, holds true today as well.Given this study was published in 2015, in terms of technological advancements across sectors; we have come a long way in IVD as well. A prominent example would be India, which recently published child and maternal health statistics, noting that considerable improvement has been observed with respect to infant and maternal mortality and morbidity1,2 – these positive changes have been made possible in part due to widespread adoption of quality diagnostics, that have evolved to become economical and quick in reporting results. Governments and regulatory agencies too have improved their processes over time, to implement important procedures in child and maternal health. The same is being observed in tuberculosis, HIV and even cancer.
How can we bridge the existing gaps to benefit patients faster and affordably?
I would like to share few thoughts on pragmatic approaches, keeping in mind, front and center, the ailing patient.
Create an accountable and time-bound process for regulatory procedures. Under the new Medical Device Regulation, many processes have been standardized and safety to patients is evaluated with rightful importance, to the credit of the authorities. However, the patient need for a cost-friendly innovative diagnostic product is lost in the regulatory procedures which could stretch from two to nine months and at times with no clear direction of where it is held up and why. A dedicated channel to assist companies with regulatory queries would help tremendously. For the procedure to be successful there needs to be accountability in terms of definitive time-bound responses for every application / submission / query from both sides, i.e. the regulatory authorities and compliance from industry.
Multiple agencies at the state and central level govern different aspects about manufacturing or research and development. MSMEs require ample resources to tend to each of those agencies from time to time, when served notices or asked queries from an agency concerning a functional area (example: consents required from a state pollution control board). Creation of dedicated Project Management Offices in establishments like NITI Aayog could assist MSMEs reach out to various agencies at state and central level and guide the companies in handling or satisfactorily completing a pending requirement to the mutual benefit of the agency and MSME. Empaneled by the government in association with the regulatory offices, it is aware of all policies, procedures, time lines and task owners. It guides the companies through the various processes and an annual fee is charged to companies for this service. This could be a win- win for all parties involved.
These initiatives would reflect a positive start and provide the momentum to start-ups and established but smaller companies in the IVD space – to build in India, for India – quicker access for the patient, affordable and product accuracy being the guiding principles.
References: