Highlights
- Human urinary albumin has been purified from the urine samples.
- Purified urinary albumin may be equivalent to a reference form of native urine and serum albumin.
- Peptide mass fingerprint analysis of urinary albumin was matched with the known sequence of ALBU_HUMAN (P02768).
- Immunoelectrophoresis infers the purified albumin appears to be homogenous and highly pure.
- This is the first report of the purification of the immunochemically reactive form of urinary albumin.

Background and aim:
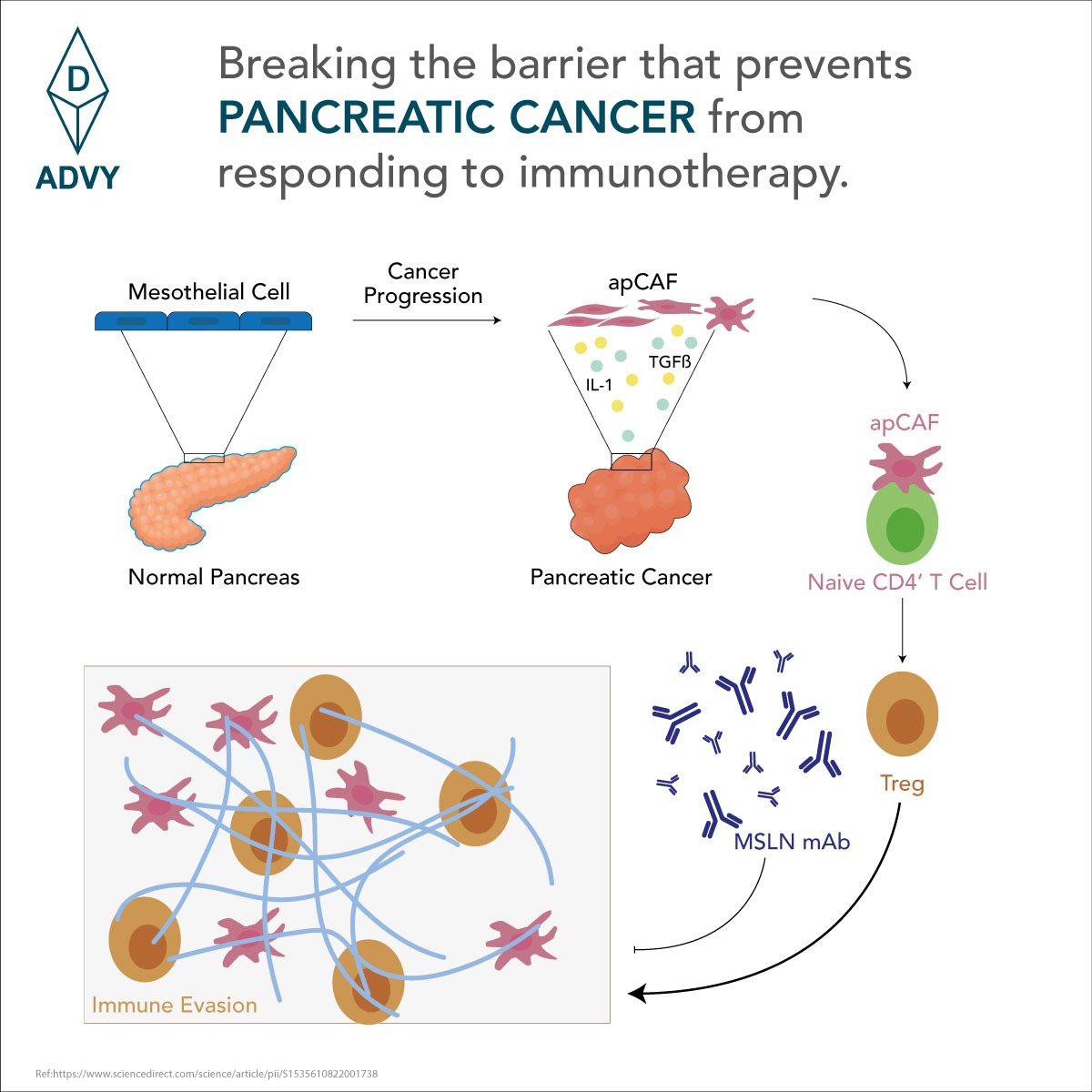
We describe a simplified approach for the purification and characterization of urinary albumin, a key biomarker currently used for understanding the onset and prognosis of microalbuminuria. Urinary albumin was purified from human urine collected from diabetic kidney disease patients by using a 2-stage tangential flow filtration process and set of column chromatography steps. The relative molecular mass of urinary albumin is 66,871 Da (SYNAPT G2 High Definition Mass Spectrometry System). Isolated urinary albumin was analyzed by SDS-PAGE, Western blotting, immunoelectrophoresis, Ouchterlony double-immunodiffusion, single radial immunodiffusion, size-exclusion HPLC, and peptide mass fingerprint analysis. The size-exclusion HPLC elution profile of the purified urinary albumin was similar to that of a reference form of native albumin. Peptide mass fingerprint analysis of the purified urinary albumin yielded peptides that partially matched with the known sequence of ALBU_HUMAN (P02768). This is the first report of purification and validation of the immunochemically reactive form of urinary albumin from a large volume of urine of diabetic kidney disease patients. In this purification approach, the cost of the purified albumin is significantly lower.
Brief Article:- Science Direct
Download Article:- Research Paper of Urinary Albumin