Background and aim:
Finch Therapeutics’ lead microbiome drug beat the standard of care at eliminating recurrent Clostridium difficile infections in a phase 2 study, laying the groundwork for a second pivotal trial and teeing up a second program in hepatitis B.
The study pitted the drug, CP101, against placebo in 206 patients who had undergone the standard-of-care antibiotics for C. difficile infection. One dose of CP101 staved off recurrent C. difficile infections for eight weeks in nearly three-quarters of patients, compared to 62% of patients who received placebo.
Finch Therapeutics’ is the second microbiome company to announce positive results for a microbe-based drug in 2020. But it is the first to do so with an oral treatment; Rebiotix announced that its Phase 3 trial for an enema-based therapy succeeded in early May 2020.
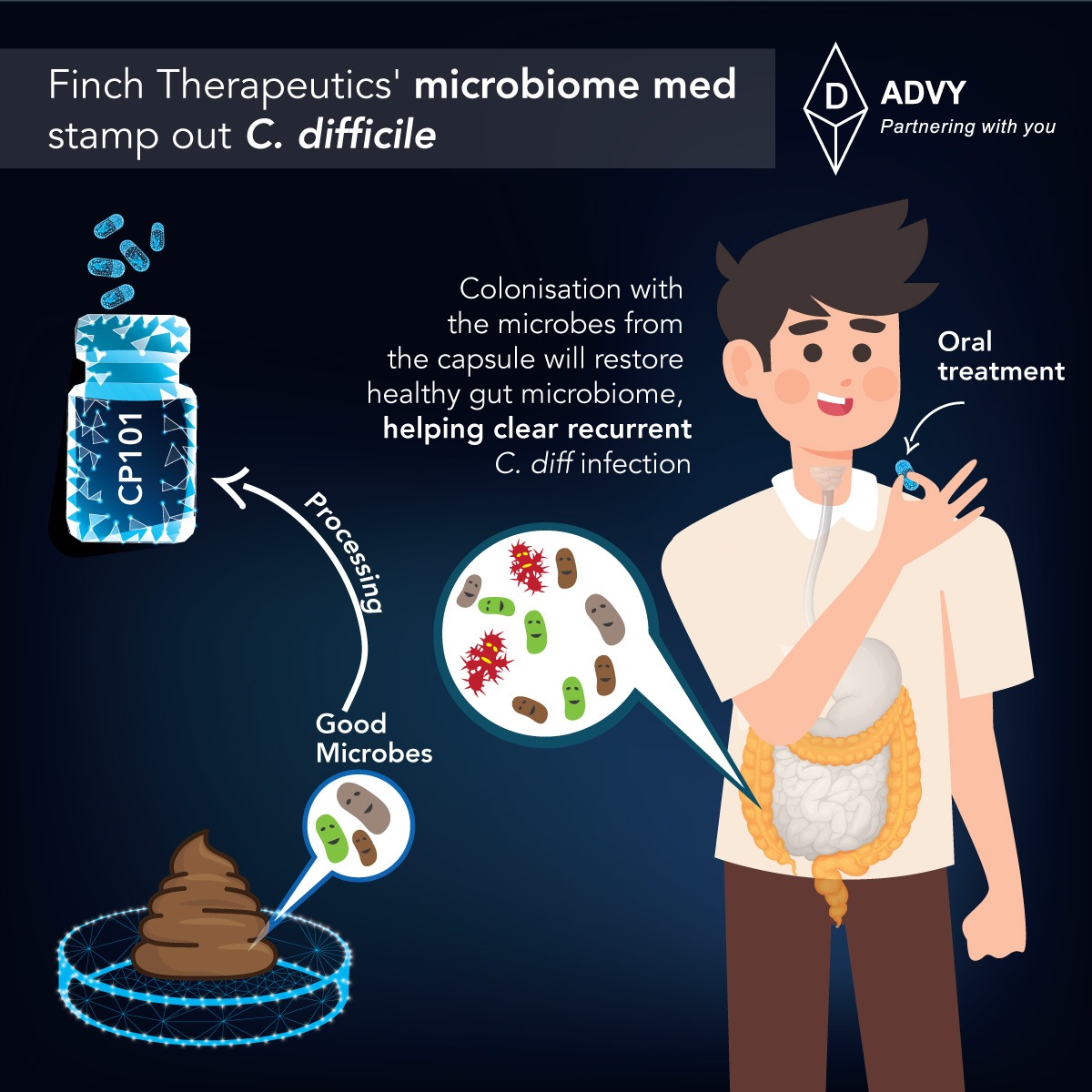

This will be an important proof point for the field,” said Finch CEO Mark Smith. “There’s a lot of people that have been excited about the promise of the microbiome and who have believed that it’s gonna be an important modality. And I think the question for a lot of people has been when and how.

Read more>> Fierce Biotech STAT