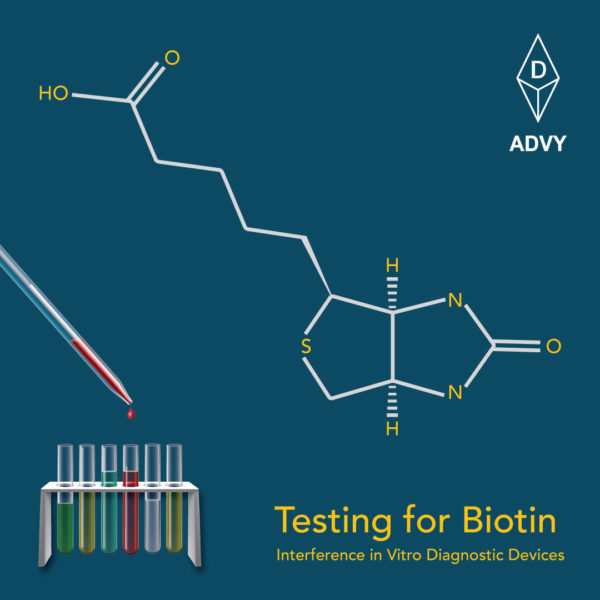
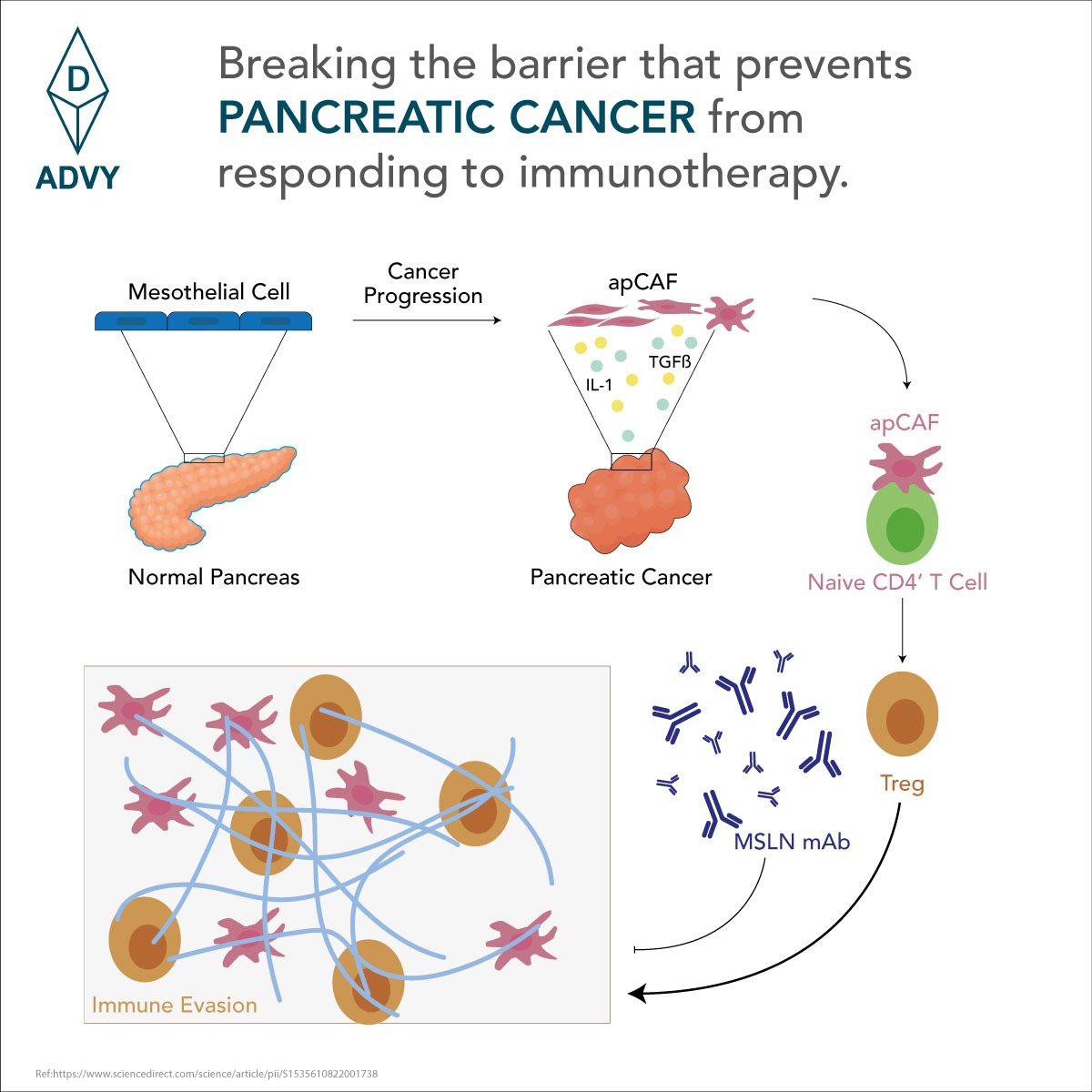
Background and aim:
US FDA has issued guidance for Biotin Interference in IVD assays. This would be helpful for clinical laboratories employing ELISA/CLIA/Other IVD assays that use biotin/avidin signal technology, which can potentially interfere with biotin in patient samples, leading to falsely high or falsely low results, depending on the test.
Reference :- US FDA